
EMA Marketing Authorization of New Drugs in November 2024
Shots:
-
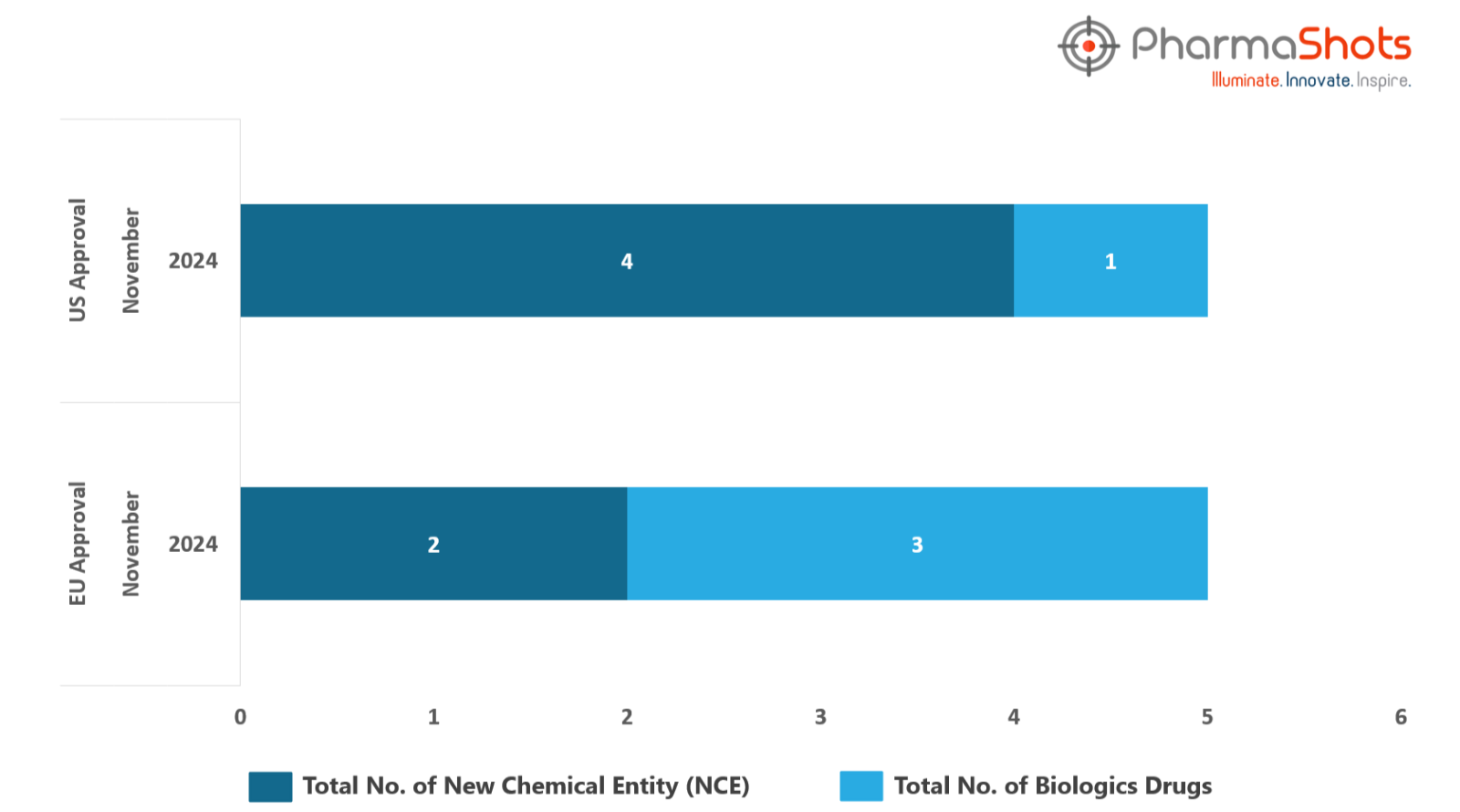
The EMA’s CHMP has granted positive opinions to 3 Biologics and 2 New Chemical Entities in November 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were AbbVie’s Elahere to treat Ovarian Cancer and Pfizer’s Hympavzi for Hemophilia A and B
-
PharmaShots has compiled a list of 5 drugs that have been granted positive opinions and approvals by the EMA’s CHMP & EC, respectively
Product Name: Augtyro
Active ingredient: Repotrectinib
Company: BMS
Date: Nov 14, 2024
Disease: NSCLC & Advanced Solid Tumors
Shots:
-
The CHMP has granted positive opinion to repotrectinib for treating ROS1+ advanced NSCLC in adults and NTRK+ advanced solid tumors in patients (≥12yrs.), with the EC’s decision anticipated in Jan 2025
-
Opinion was based on P-I/II (TRIDENT-1 & CARE) trials, with TRIDENT-1 assessing repotrectinib in advanced solid tumors (NSCLC and tumors with ROS1 & NTRK fusions), while CARE focuses on pediatric & young adults with ALK, ROS1, or NTRK gene alterations
-
Trials showed meaningful response rates in the patients along with strong durable responses and intracranial activity; safety profile was manageable with standard treatments. Study continues to assess long-term outcomes and additional EPs
2. InflaRx’s Gohibic (Vilobelimab) Gains the CHMP’s Positive Opinion for the Treatment of ARDS
Product Name: Gohibic
Active ingredient: Vilobelimab
Company: InflaRx
Date: Nov 14, 2024
Disease: SARS-CoV-2-Induced Acute Respiratory Distress Syndrome
Shots:
-
The CHMP has recommended Gohibic (under exceptional circumstances) to treat SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) adults on systemic corticosteroids (SoC) & IMV (with/without ECMO), with the decision anticipated within 67days (Q1’24)
-
Opinion was backed by P-III (PANAMO) study of Gohibic vs PBO among invasively mechanically ventilated COVID-19 patients in ICU, showing improvement in survival with a 23.9% relative reduction in 28-day all-cause mortality. Data was published in The Lancet Respiratory Medicine
-
The company is seeking commercial distribution partnerships in the EU for Gohibic (mAb targeting human complement factor C5a)
Product Name: Rybrevant + Lazcluze
Active ingredient: Amivantamab + Lazertinib
Company: Johnson & Johnson
Date: Nov 14, 2024
Disease: EGFR-Mutated NSCLC
Shots:
-
The CHMP has recommended Rybrevant + Lazcluze as a 1L treatment of NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and approval of Type II extension of indication for amivantamab
-
Opinion was based on the P-III (MARIPOSA) study assessing Rybrevant + Lazcluze vs osimertinib & Lazcluze in NSCLC patients (n=1,074)
-
Trial achieved its 1EP, showing a 30% reduced disease progression or death risk (mPFS: 23.7 vs 16.6mos.) at 22mos. follow-up & mDoR of 25.8 vs 16.8mos. It also included serial brain MRIs to detect CNS events & depicted mPFS of 27.5 vs 18.4mos.; long-term follow-up (31.1mos.) showed a favorable OS trend, with 61% vs 53% alive, mOS not estimable [NE] vs 37.3mos.
Product Name: Hympavzi
Active ingredient: Marstacimab
Company: Pfizer
Date: Nov 20, 2024
Disease: Hemophilia A and B
Shots:
-
The EC has approved Hympavzi (QW, SC) as a prophylactic treatment to prevent bleeding episodes in patients (≥12yrs., at least 35kg) with hemophilia A & B, without FVIII & FIX inhibitors, respectively. It is valid across the EU plus Iceland, Liechtenstein & Norway
-
Approval was based on pivotal P-III (BASIS) study of Hympavzi in patients (12-75yrs.) with severe hemophilia A or mod. severe to severe hemophilia B with/without inhibitors
-
Study showed a reduction in the annualized bleeding rate (ABR) by 35% (5.08 vs 7.85) during 12mos. with Hympavzi vs routine prophylaxis & on-demand treatment. Safety aligned with the P-I/II study, with most common AEs being injection site reactions, headache, pruritus & hypertension
Product Name: Elahere
Active ingredient: Mirvetuximab Soravtansine
Company: AbbVie
Date: Nov 14, 2024
Disease: Ovarian Cancer
Shots:
-
The EC has approved Elahere to treat FRα+, Pt-resistant high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer after 1-3 prior treatments, valid across whole EU plus Iceland, Liechtenstein, Norway & Northern Ireland
-
Approval was based on P-III (MIRASOL) trial assessing Elahere vs investigator's choice of CT (weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan) in patients (n=453) to treat FRα+, Pt-resistant, high-grade serous ovarian cancer
-
Results showed reduction in progression or death risk by 35% & reduction in death risk by 33%. Common AEs included blurred vision, nausea & fatigue, with pneumonitis as the most serious one. Data was published in the NEJM
Related Post: Insights+: EMA Marketing Authorization of New Drugs in October 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














